
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoMethods of preventing corrosion: (i). Alloying:
Metals can be alloyed to prevent the corrosion process.
Example:
Stainless Steel
(ii). Surface coating:

Surface coating process
Surface coating involves the application of a protective layer over the metal. It is classified as follows.
(a). Galvanization:
Galvanization is a method of coating zinc on iron sheets by using an electric current.

Industrial galvanization process
(b). Electroplating:
Electroplating is a technique of coating one metal over another metal by passing an electric current.
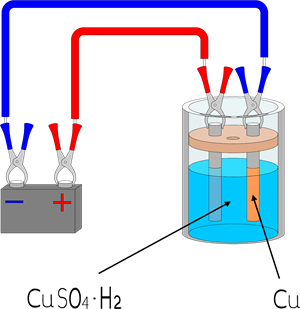
Copper electroplating technique
(c). Anodizing:
Anodizing is an electrochemical method that turns the metal surface into decorative, durable and corrosion-resistant. Aluminium is widely used for the anodizing process.
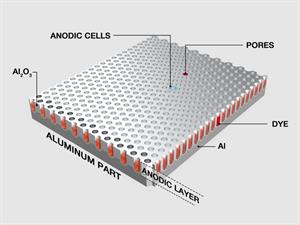
Aluminium anodizing process
(d). Cathodic protection:
Cathodic protection is a process of controlling corrosion of a protected metal surface coated with easily corrodible metal. The easily corrodible metal is called sacrificial metal, which acts as an anode, ensuring cathodic protection.
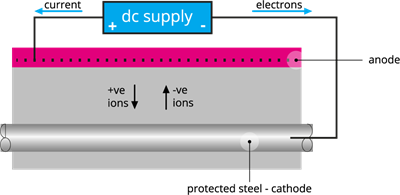
Steel cathodic protection process