PDF chapter test TRY NOW
Substances exist in three physical states (phases), namely solid, liquid, and gas.
States of matter
Let us see the general characteristics of states of matter:
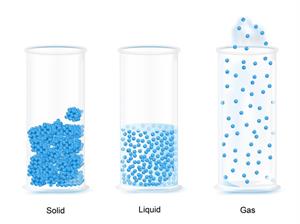
Packing of molecules
Solids:
- In solid, the molecules are closely packed.
- They have definite shape and volume.
- Due to their arrangement, they have a strong intermolecular force and less intermolecular space.
Liquid:
- In liquid, the molecules are loosely packed.
- They do not have a definite shape, but they do have a definite volume.
- Due to their arrangement, they have a less intermolecular force and more intermolecular space compared to solids.
Gases:
- In gases, the molecules are very loosely packed.
- They do not have a definite shape and volume.
- Due to their arrangement, they have a very weak intermolecular force and a very large intermolecular space.
Both the solvent and the solute in binary solutions can exist in any form of the above mentioned physical states.
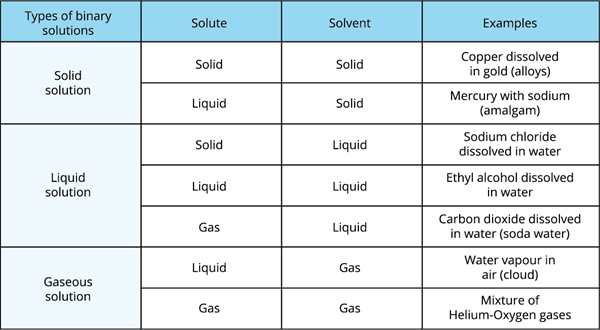
Different types of binary solutions
However, the solvent accounts for the majority of the solution. The physical state is the primary factor that determines the characteristics of the solution.
