PDF chapter test TRY NOW
Compressibility is the measure of reducing the volume of a substance when pressure is applied to it.
Gases are highly compressible compared to solids and liquids due to the larger space between the particles (or ions or molecules) in them. For example, see the figure given below:
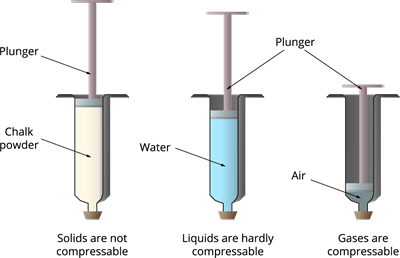
Consider three syringes filled with chalk powder, liquid and gas. When you press the plunger down (where the pressure is applying), the following changes will occur:
A syringe with chalk powder: Cannot be compressed since the solid particles are packed tightly.
A syringe with liquid: Hardly compressible since they can move and keep the same volume.
A syringe with gas: Highly compressible since they have a large amount of empty spaces.
A syringe with chalk powder: Cannot be compressed since the solid particles are packed tightly.
A syringe with liquid: Hardly compressible since they can move and keep the same volume.
A syringe with gas: Highly compressible since they have a large amount of empty spaces.
Difference between the state of solids, liquids and gases:
| S.No. | Properties | Solid | Liquid | Gas |
| 1. | Arrangement of particles | Regular arrangement | No regular arrangement | No regular arrangement |
| 2. | Volume | Definite volume | Definite volume | No definite volume |
| 3. | Shape | Definite shape | No definite shape | No definite shape |
| 4. | Density | Affected by density | Affected by density | Not affected by density |
| 5. | Diffusion | Minimum | Minimum | Maximum |
| 6. | Force of attraction | Maximum | Maximum | Minimum |
| 7. | Moment of particles | Cannot move | Slowly moves | Highly moves |
| 8. | Compressibility | Not compressible | Hardly compressible | Highly compressible |
Liquefaction:
The process of converting gaseous state substance into the liquid state by applying pressure on the gaseous substance is known as liquefaction.
When pressure is applied, gas molecules come closer and reduce the temperature, removing sufficient energy to convert from gas to liquid.
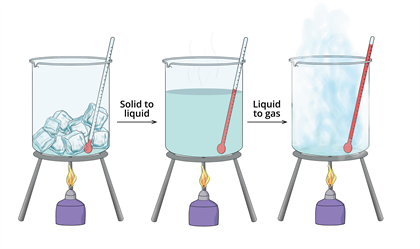
The following image shows the process of converting solid to liquid state and liquid to gaseous state by increasing the temperature. Meanwhile, decreasing the temperature will reverse the process.