
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoLet's see what an atom, molecule and elements are!
Atoms make up everything that you can see, touch, smell, feel, and taste.
Atoms are the essential building blocks of all matter (including you and anyone else you'll ever meet). So, let us learn a few things about these extraordinarily tiny particles if we want to understand more on this topic.

Everything you've ever seen is made up of atoms or atoms stuck together in dizzyingly complex configurations.

A chemical element is a material that cannot be broken down into simpler forms using traditional chemical methods.
Molecules of compounds contain atoms from two or more separate elements. For example, Ethane C_2H_6 has eight atoms, two of carbon C and six of hydrogen.
An atom is the smallest unit of a chemical element. Atoms are incredibly tiny, smaller than anything we can imagine or compare them to.
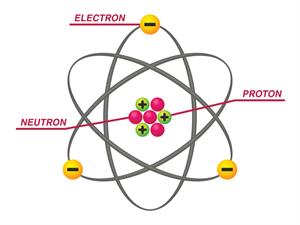
Parts of Atoms:
Subatomic particles (sub- means "smaller size") are the particles that make up an atom. These particles are the
- Proton (p^+), a positively + charged particle;
- Electron (e^–), a negatively – charged particle;
- Neutron (n^0), a neutral particle with no charge 0.
The nucleus, or core, of the atom, is made up of protons and neutrons. Outside of the nucleus of the atom, electrons live in shells.
Electrostatic forces hold atoms in molecules together, such as the two hydrogen atoms in H_2 gas.
What's Inside an Atom?