
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoA molecule is the smallest particle of an element or compound capable of an independent life and exhibiting all of the substance's properties.
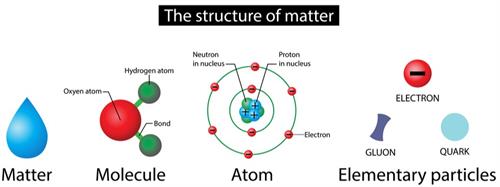
In general, a molecule is a set of two or more atoms that are chemically bound or held together by attractive forces.
Atoms from the same or different elements may combine to form a molecule.
Atoms from the same or different elements may combine to form a molecule.
Example:
- To understand it, consider a water molecule, the two elements, hydrogen and oxygen atoms combined, to form a water molecule.
- Calcium oxide CaO contains two elements, calcium and oxygen atoms combined to form a calcium oxide molecule.
- Oxygen O_2 contains two elements of an oxygen atom.
- Chlorine Cl_2 contains two elements of a chlorine atom.
Molecules of elements:
The atoms that make up an element's molecules are all of the same nature.
- Monoatomic Elements:

In their molecular form, certain elements are monatomic, meaning they are made up of just one atom (mono-atomic).
Helium He is an example of a monoatomic element.
- Diatomic Elements:
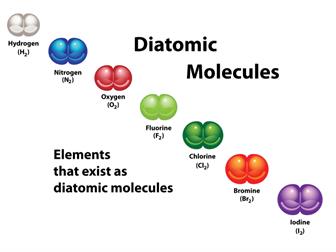
If the molecule constitutes two atoms, we can classify it as a diatomic molecule. In their molecular shape, other elements have two or more atoms.
Each molecule of hydrogen H_2, oxygen O_2, and chlorine Cl_2, for example, has two atoms.
- Triatomic Elements:
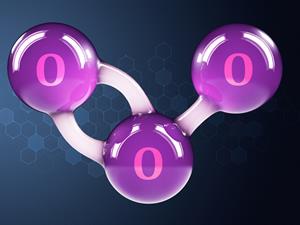
If another atom is combined with , it forms ozone, which is a triatomic molecule.
Note:
- Monoatomic and diatomic molecules are more stable than triatomic molecules.
- Most non-metal elements are diatomic molecules.
Molecules of compounds:
Compound molecules contain atoms from two or more separate elements.
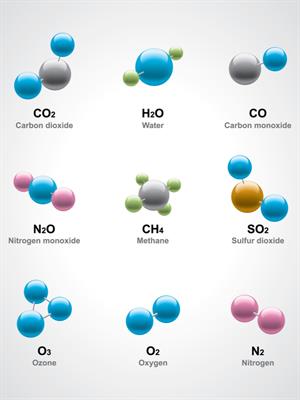
Example:
Water H_2O, for example, has three atoms: Two hydrogen (H) atoms and one oxygen (O).

Methane CH_4 with five atoms: One carbon (C) and four hydrogen (H) atoms.
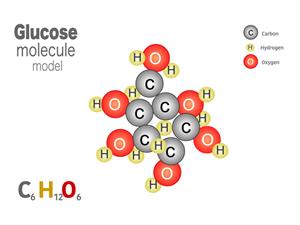
Glucose C_6H_{12}O_6 contains elements such as 6 carbon, 12 hydrogen and 6 oxygen, combined to form a glucose molecule.
Sodium chloride NaCl contains the one element of each sodium and chlorine combined to form a sodium chloride molecule.