
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoCoordinate Covalent bond or Dative bond:
In the formation of a normal covalent bond, each of the two bonded atoms contributes one electron to construct the bond.
A covalent bond is formed between two atoms by sharing two electrons, both of which come from combining atoms. This type of bond is known as a Coordinate covalent bond or a Dative bond.
- The lone pair of electrons from an atom in a molecule is usually involved in dative bonding.
- The atom that provides the electron pair is known as the donor atom, while the atom that accepts the electron pair is known as the acceptor atom.
- An arrow () leading from the donor to the acceptor atom represents a coordinate covalent bond.
Formation of coordinate covalent bond:
Consider two atoms, A and B. Assume that atom A has an unshared lone pair of electrons and atom B lacks two electrons in its valence shell.
Atom A now donates its lone pair, accepted by atom B.
Thus, the lone pair of electrons formerly belonged to atom A is now given by both the atoms, and the bond made by this mutual sharing is called a Coordinate covalent bond A B.

Coordinate covalent bond formation
Example:
(NH_4{^+}, NH_3 BF_3)
Illustration 1- Formation of a coordinate covalent bond between NH_3 → BF_3 molecules:
- The donated pair of electrons arrive from an already made molecule to another acceptor molecule.
- In this case, the molecule ammonia (NH_3) contributes a single pair of electrons to the electron-deficient molecule boron trifluoride (BF_3).
- Thus, NH_3 (donor molecule) and BF_3 (acceptor molecule) form a coordinate covalent bond, which represent NH_3 → BF_3.
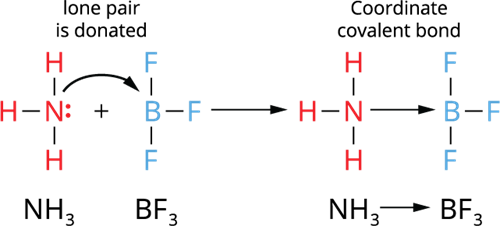
Example of coordinate covalent bond