PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoCorrosion:
When you slice an apple and leave it for a while, the outside layer of the apple turns brown. Furthermore, iron bolts and nuts in metallic structures corrode in a similar fashion.
If you have any clue why this is happening?
It is due to a chemical reaction known as oxidation.

Corrosion on car
Oxidation:
The chemical reaction includes the addition of oxygen or removal of hydrogen or loss of electrons is called Oxidation.
Example:
2Mg + O_2 → 2MgO (addition of oxygen)
CaH_2 → Ca + H_2 (removal of hydrogen)
Fe^2{^+} + → Fe^3{^+} + e^− (loss of electron)
Fe^2{^+} + → Fe^3{^+} + e^− (loss of electron)
Reduction:
The chemical reaction includes the addition of hydrogen or removal of oxygen or gain of electrons is called Reductions.
Example:
2Na + H_2 → 2NaH (addition of hydrogen)
CuO + H_2 → Cu + H_2O (removal of oxygen)
Fe^3{^+} + e^− → Fe^2{^+} (gain of electron)
Fe^3{^+} + e^− → Fe^2{^+} (gain of electron)
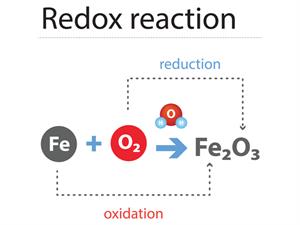
Redox reactions:
The oxidation and reduction happen in a similar reaction (simultaneously).
If one reactant gets oxidised, the other gets reduced. Such reactions are named as Oxidation-reduction reactions or Redox reactions.
Example:
2PbO + C → 2Pb + CO_2 (reduction)
Zn + CuSO_4 → Cu + ZnSO_4 (oxidation)
Zn + CuSO_4 → Cu + ZnSO_4 (oxidation)