PDF chapter test TRY NOW
A chemical compound is formed when two distinct elements are chemically combined i.e. chemical bonds form between their atoms.
Example:
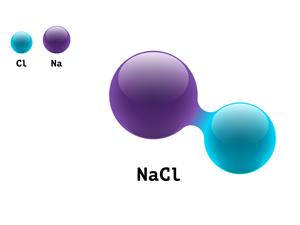
Salt NaCl
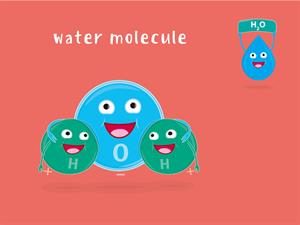
WaterH_2O
Ozone O_3, Nitrogen dioxide NO_2, and so on.
Compounds are a form of matter created by combining two or more elements in a specific mass ratio. Chemical methods may be used to decompose it into its constituent components.
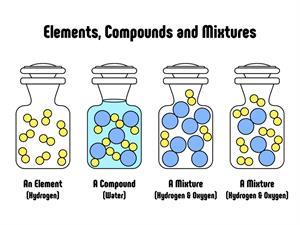
Properties of Compounds:
- Two or more elements are chemically mixed in a compound.
- The elements in a compound are present in a fixed mass ratio.
- Physical methods cannot isolate the constituents of a compound.
- The constituents of a compound lose their identities, i.e., A compound's properties vary from those of its constituent elements.
Example:
Constituent elements in a compound:
Water (H_2O) has three atoms: Two hydrogen (H) atoms and one oxygen (O) atom.

Methane CH_4 with five atoms: One carbon (C) atom and four hydrogen (H) atoms.
Glucose C_6H_{12}O_6 contains elements of carbon 6, hydrogen 12 and oxygen 6 combined to form a glucose molecule.

Sodium chloride NaCl contains the two elements: sodium and chlorine atoms combined to form a sodium chloride molecule.
