
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free Demo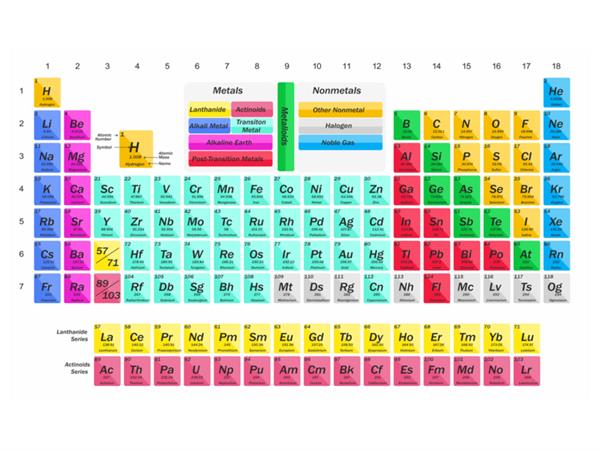
Benefits:
- The table is based on the atomic number, which is an essential property.
- It more precisely relates the element's location to its electronic configuration.
- Each period's conclusion is more logical. The energy shells progressively fill up as the atomic number increases until an inert gas configuration is reached.
- It's easy to note and repeat.
- Each group is self-contained, and the concept of sub-groups has been discarded.
- Since all isotopes of an element have the same atomic number, one position for all isotopes is justified.
- In this table, the eighth group's location (in Mendeleev's table) is also justified. Since the properties of transition elements are intermediate between the left and right portions of the periodic table, they have been grouped in the middle.
- Metals and non-metals are fully separated in the table. The non-metals are found in the periodic table's upper right corners.
- Since it depends on the atomic number of the elements, the locations of those previously interchanged elements in Mendeleev's periodic table are now justified.
- The placement of lanthanides and actinides at the bottom of the periodic table has been defended.
Hydrogen position in the periodic table:
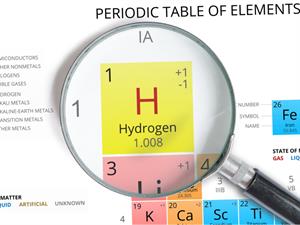
The lightest, smallest, and the first element in the periodic table is hydrogen. Its electronic configuration is (1s^1). It holds a unique position in the periodic table. It has properties that are similar to both alkali metals and halogens.
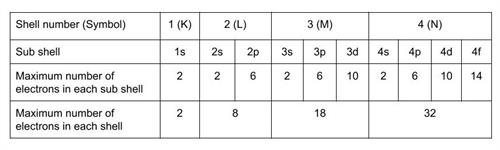
The number of electrons in a subshell:
- Alkali metals are placed on the top periodic table.
- Hydrogen, like alkali metals, can lose one electron to form a hydrogen ion (H^+).
- It can also obtain one electron to form the hydride ion (H^-) like halogens.
- Hydrogen is a gas, whereas alkali metals are solids.
Hence, hydrogen in the modern periodic table is still under discussion as the properties of hydrogen are unique.